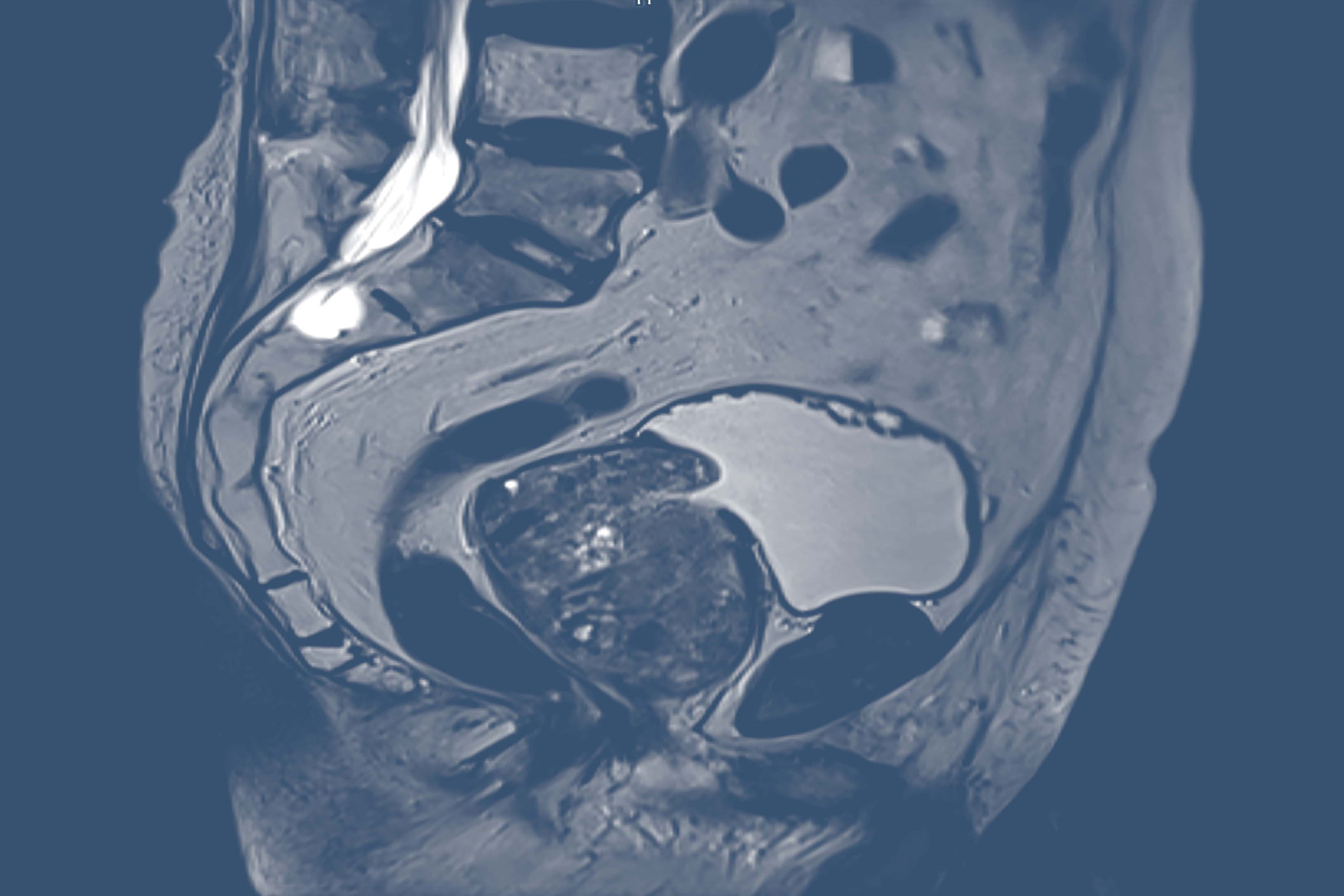
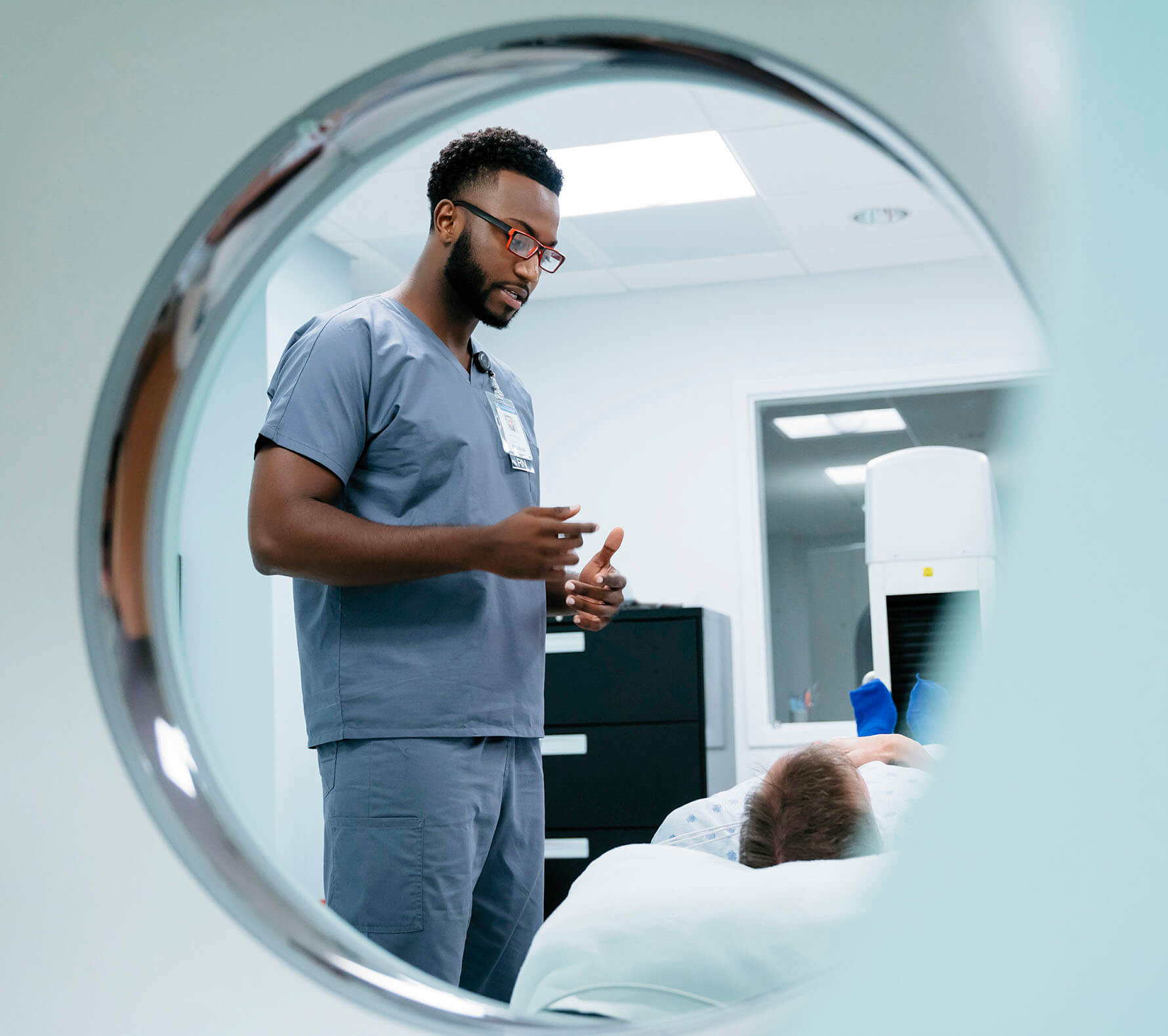
Because prostate cancer diagnosis and treatment must advance
Since its founding in 2014, Blue Earth Diagnostics has been focused on addressing unmet needs in prostate cancer.
While there have been many advances in screening, diagnosis, and management in recent years, prostate cancer remains a leading cause of new cancer cases in men in the United States.1,2 Various diagnostic imaging agents are utilized throughout the continuum of care.3-6 However, conventional imaging techniques have many limitations when it comes to prostate cancer identification and localization.6-13 Greater imaging accuracy is needed, at the earliest possible stage of disease and throughout the care continuum, to optimize therapeutic decision-making.
Blue Earth Diagnostics is unlocking the potential of radiopharmaceutical technology with the goal of changing the future of prostate cancer care.
Advancing research
Our Investigator-Initiated Trials are managed by qualified independent medical researchers who have full responsibility for the study and assume the obligations of a sponsor—through proposal development, protocol finalization, study conduct and analysis, and dissemination of results.
Blue Earth Diagnostics partners with world-renowned research institutions to develop and study promising compounds with the potential to be next-generation solutions for patients with prostate cancer.
Some of our research alliances include:



Encouraging diversity in clinical trials
Blue Earth Diagnostics is dedicated to promoting inclusive participation in our clinical trials, particularly within the African American community, to reduce health disparities and improve care. Given the high prevalence and mortality of prostate cancer among African American men, a post hoc analysis was conducted to measure the diagnostic performance of a PET agent in African American patients.14,15

Empowering patients with education and inspiration
Patients and caregivers are at the heart of all that we do. We partner with the following advocacy, societal, and community outreach organizations to support patients with prostate cancer.


Empowering patients with education and inspiration

Patients and caregivers are at the heart of all that we do. We partner with the following advocacy, societal, and community outreach organizations to support patients with prostate cancer.
If you would like to learn more about our advocacy activities, email us here.
Learn what to expect during a PET scan
The more you know about how a PET scan is administered, the better prepared you’ll be.
References
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Prostate Cancer. Accessed October 13, 2021. https://seer.cancer.gov/statfacts/html/prost.html.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021 [published correction appears in CA Cancer J Clin. 2021 Jul;71(4):359]. CA Cancer J Clin. 2021;71(1):7-33.doi:10.3322/caac.21654
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Guideline for Prostate Cancer (Version v1.2022). © National Comprehensive Cancer Network, Inc. 2021. All rights reserved. Accessed September 17, 2021. To view the most recent and complete version of the guideline, go to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Trabulsi EJ, Rumble RB, Jadvar H, et al. Optimum imaging strategies for advanced prostate cancer: ASCO guideline. J Clin Oncol. 2020;38(17):1963-1996. doi:10.1200/JCO.19.02757
- Expert Panel on Urologic Imaging; Coakley FV, Oto A, Alexander LF, et al. ACR Appropriateness Criteria® Prostate Cancer-Pretreatment Detection, Surveillance, and Staging. J Am Coll Radiol. 2017;14(5S):S245-S257. doi:10.1016/j.jacr.2017.02.026
- Expert Panel on Urologic Imaging; Froemming AT, Verma S, Eberhardt SC, et al. ACR Appropriateness Criteria® Post-treatment Follow-up Prostate Cancer. J Am Coll Radiol. 2018;15(5S):S132-S149. doi:10.1016/j.jacr.2018.03.019
- Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179(3):906-910. doi:10.1016/j.juro.2007.10.059
- Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective [published correction appears in Radiology. 2007 Oct;245(1):302]. Radiology. 2007;243(1):28-53. doi:10.1148/radiol.2431030580
- Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50(6):1163-1174. doi:10.1016/j.eururo.2006.06.025
- Schiavina R, Ceci F, Borghesi M, et al. The dilemma of localizing disease relapse after radical treatment for prostate cancer: which is the value of the actual imaging techniques? Curr Radiopharm. 2013;6(2):92-95. doi:10.2174/1874471011306020005
- Wolf JS Jr, Cher M, Dall’era M, Presti JS Jr, Hricak H, Carroll PR. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol. 1995;153(3 pt 2):993-999.
- Merdan S, Womble PR, Miller DC, et al. Toward better use of bone scans among men with early-stage prostate cancer. Urology. 2014;84(4):793-798. doi:10.1016/j.urology.2014.06.010
- Ikonen S, Kärkkäinen P, Kivisaari L, et al. Magnetic resonance imaging of clinically localized prostatic cancer. J Urol. 1998;159(3):915-919.
- Giaquinto AN, Miller KD, Tossas KY, et al. Cancer statistics for African American/Black people 2022. CA Cancer J Clin. 2022;72(3):202-229. doi:10.3322/caac.21718.
- Rais-Bahrami S, Fleming M, Gartrell B, et al. 18F-flotufolastat positron emission tomography in African American patients with suspected prostate cancer recurrence: findings from the phase 3 SPOTLIGHT study. Adv Radiat Oncol. 2024;9(9):101571. doi:10.1016/j.adro.2024.101571